Why is stainless steel stainless?
Iron vs Steel
Steel is made from iron, but it’s not the same thing: steel is an alloy - iron doped with other elements to engineer new, useful properties. Some of these elements have been especially selected to provide certain properties, but not all metallurgy is that well understood: some elements have simply been stuck in and performed well - and we don’t know why.
The key element that makes iron into steel is carbon. Pure iron is easy to shape, stretch and work, and may even be cut with a knife with a bit of effort. Carbon allows steel to be heat-treated, making it harder and stronger than iron. The more carbon, the harder and stronger the steel becomes. However, it also becomes more brittle, easily snapped and harder to shape. The ideal amount of carbon in steel is less than 2%. Iron doped with more than 2% carbon is considered inferior and referred to as “pig iron”.
Even better than steel... is stainless steel. Stainless steel has a massive advantage over ordinary, carbon steel and iron because it is highly rust resistant. Elemental iron is very vulnerable to corrosion, rusting rapidly in moist air. This makes it dangerous for construction, ships, and household items like boilers. Iron can be made stainless by adding chromium (at least 16%). Chromium is even more reactive than iron in air, and quickly forms a chromium oxide (Cr2O3) at the surface. Although this initially seems like a disadvantage, the rusting (or oxidation) is so fast and uniform it creates a protective oxide layer or passive film. This acts as a barrier, preventing the steel beneath from rusting.
Stainless steel does still corrode a little bit. This happens when the passive film is damaged in some way, like being scratched or broken down chemically. Chemicals that destroy the passive layer include chloride ions, which are found in common salts and seawater. Corrosion may be measured by measuring charge across the metal surface: as atoms oxidise to form charged ions, a current passes along the surface. These oxidised atoms can then either dissolve (in the moisture in the air, rainwater or ocean), or bind with oxygen to form a new passive film.
Staining Stainless Steel
Normally, the passive film on the surface of a stainless steel object is formed when a water molecule sticks to an ion on the surface, then bonds by releasing an H+ ion to make a surface hydroxide. This can then go on to lose another H+ to become an oxide. This oxide can stay stuck to the surface or dissolve into solution depending on how strongly the metal it is bound to is attached to other metal atoms.All sorts of things react with the surface of a metal, not only water. Surfaces are very complex and covered in all kinds of things - this is why surface science is its own branch of chemical physics.
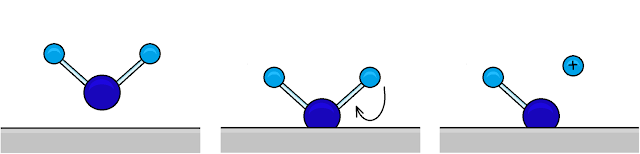 |
| A water molecule adheres to a metal surface by binding to a positively charged metal ion within it. It then loses an H+ ion to form a stronger bond with the surface. Image ©TWDK. |
Most steels have a crystalline structure made up of more than one alloy, or compound, mixed together. This takes on the appearance of chunks of chocolate in a cookie, where the chunks are one steel alloy and the cookie is another. These steels are called austenite and ferrite. Both the different elements and how they are bonded determine the properties of the steel. Austenite contains ~18% chromium, 8% nickel, and smaller amounts of molybdenum and nitrogen, whilst ferritic steels have a low carbon content and chromium ~10.5-27%. As a result, the metal corrodes unevenly.
Mo - Why don’t we know?
Elements other than chromium, such as molybdenum and nitrogen, are added to further discourage corrosion, or to adapt the physical and engineering properties of the steel. They sometimes change the oxidation states of the other metals, and sometimes affect reactivity. For example, nickel is added to make chromium-toughened steel more malleable. It doesn’t contribute to the passive film, but surface analyses have still found it enriched at the surface - why this is or what it is doing there, we don’t know[1].Another element found enriched at the surface is molybdenum[1]. Molybdenum, Mo, is a transition metal. Sitting below chromium in the periodic table of elements, it has similar properties and behaves similarly. So it is not unexpected to discover that molybdenum is
one of the most potent alloying additions for improving corrosion resistance[1, 2]. Adding just a small amount of molybdenum to steel means that less charge is passed across the surface before the system “switches off” in tests done in the presence of passive-film-eating chloride ions - i.e. the formation of the protective passive film has been accelerated. Researchers have worked out that molybdenum saves three layers of atoms from being dissolved[3]. This isn’t very much - perhaps half a nanometre thick - but it’s a lot compared to other inhibitors. Yet strangely, although many studies have investigated the process by which molybdenum halts corrosion, this mechanism remains nebulous.
Competing theories
Several theories have been proposed to explain the effectiveness of molybdenum in corrosion resistance. The three main hypotheses are (1) oxide enrichment of the passive film, (2) inhibition of active dissolution, and (3) the formation of halide-harvesting polymeric molybdate hydrates.- The oxide enrichment theory proposes that molybdates form with Mo4+ or Mo6+ ions, such as MoO2, contributing directly to the passive film. This film is commonly described by the Okamoto model, with an inside and an outside, or duplex structure[1]. In this theory, the molybdates form part of the inner layer of the passive film, in contact with the metal. The inner film acts as a diffusion barrier, stopping metal ions from dissolving out and metal-eating chloride ions from coming in. The theory suggests that oxide enrichment enhances the stability of the passive film. One way it might do this is by replacing chloride ions in the film with negatively charged molybdates, so decreasing the number of chloride ions. Chloride ions in the film mean aggressive chloride ions closer to the metal surface, which is not good - especially as they form very few bonds, creating defects in the structure that other ions can travel through. So if molybdenum is removing the number of chloride ions, the film will be more robust.
- The inhibition of active dissolution theory is also a surface enrichment theory. However, in this theory, molybdenum doesn’t stabilise the passive film chemically, but blocks the surface by occupying kinks and steps in it. Even a very smooth looking surface has kinks and steps on an atomic level, where bulky things can get trapped. In this theory, it doesn’t matter what form molybdenum is in - just that it sticks on the surface. The solubility of metal ions is determined by their size and oxidation states. Molybdenum dissolves as Mo3+, but is normally slower to dissolve than iron, leading to its surface accumulation[3]. Once all over the surface, the molybdenum gets in the way of dissolving ions beneath, slowing them down and eventually stopping them[4]. This speeds up the formation of a passive film.
- The polymeric molybdate hydrates [MoO4(H2O)2]2- theory describes a network of oxygen- and hydroxide-interlinked Mo6+ centres that form exclusively in the outer layer of the passive film[5]. Some may also form solids with chromium oxides and hydroxides in the film. Molybdenum then does not dissolve and contribute to the corrosion, and in addition, chloride ions (or other halides) are taken up into the polymer, and trapped in specific places. This means there are fewer free chloride ions to break down the passive film: the pH goes up, and so the solution is less acidic and less corrosive.
New Evidence
In 2011, an experiment was performed using XANES to look for evidence for the third theory of polymobdates[4]. XANES, X-ray Absorption Near Edge Structure, is an analytical technique where x-rays are used to investigate the oxidation states of elements in compounds. It works by providing energy that incites electronic transitions within atoms, and the amount of this energy is mapped in spectra that have distinctive shapes depending on their chemistry. This experiment was looking at the oxidation states of molybdenum, and interestingly found Mo3+, the common dissolution product of molybdenum metal, but no Mo6+. This meant that Mo6+ polymobdates couldn’t have formed, and couldn’t be causing the corrosion inhibition.Two other theories remain, and although there is evidence for both, there isn’t any evidence to disprove either. It is also possible that both mechanisms are operating simultaneously, although one mechanism might be dominant over the other. Nevertheless, scientists working with one theory or the other have invested their time, funding and expertise into investigating it, and until one hypothesis is definitively disproved, they will have to coexist harmoniously.
why don't all references have links?
[1] Clayton, Clive R., and I. N. G. E. M. A. R. Olefjord. "Passivity of austenitic stainless steels." Corrosion mechanism in theory and practice, Marcel Dekker, NY (1995).
[2] A. J. Sedriks, Corrosion of Stainless Steels, Wiley, New York, 1979
[3] Newman, R. C. "The dissolution and passivation kinetics of stainless alloys containing molybdenum—1. Coulometric studies of Fe Cr and Fe Cr Mo alloys." Corrosion Science 25.5 (1985): 331-339. doi: 10.1016/0010-938X(85)90111-8
[4] Davenport, Alison J., et al. "XANES study of the chemistry of molybdenum in artificial corrosion pits in 316L stainless steel." Journal of The Electrochemical Society 158.5 (2011): C111-C117. doi: 10.1149/1.3559457
[5] Kimura, Masao, Michio Kaneko, and Noriaki Ohta. "In situ analysis of pitting corrosion in artificial crevice of stainless steel by X-ray absorption fine structure." ISIJ international 42.12 (2002): 1399-1403. doi: 10.2355/isijinternational.42.1399

No comments:
Post a Comment