Since the publication of Charles Darwin’s seminal book On the Origin of Species in 1859, biologists have been looking back through the evolutionary chain to figure out what early life forms all species are descended from. From the mid-20th century, great advances in molecular biology have allowed us to determine what the earliest single-celled organisms were like, but how these life forms developed - the origin of life itself - is still largely a mystery.
There are many questions yet to be answered about the origin of life. What were the first self-replicating components - DNA, RNA or something else entirely? What materials and conditions on the early Earth were present to build these components? And why is it that these and the other building blocks of life are all homochiral - that is, why are their molecules all structured in the same way, and not a mixture of the different ways possible?
To understand homochirality, we first need to look at the most basic building blocks that make up all lifeforms. The body of any living creature is made up of molecules – groups of atoms bonded together in a useful way. These range from the very small, such as water made from only three atoms, to the very large, such as the DNA strands that make up our genetic code.
Many of the big molecules in living creatures are made of smaller units joined together: starch, a carbohydrate found in food, is made from the sugar glucose, and proteins are made up of many different amino acids.
Molecules that have the same number and type of atoms but joined together in different ways are called “isomers” of each other. For example, depending on how the atoms are arranged, the C3H6O (that is, 3 carbon, 6 hydrogen and one oxygen atom) could be acetone, found in nail varnish remover, or methyl vinyl ether, used to make plastic, or also allyl alcohol used to make glycerol, which is found in many medicines and foodstuffs.
It's a special kind of isomerism, called stereoisomerism, that is important when discussing homochirality. Stereoisomerism can occur when a molecule has a carbon atom joined to four different groups. Remember that molecules aren't really flat like the drawings above, but have a three-dimensional structure. Stereoisomers have the same atoms bonded together in the same way, but are mirror images of each other. Think of your hands – the fingers, thumb and palm are joined together in the same way on each hand, but in opposite directions. In fact, molecules which can have stereoisomers are called “chiral,” from the ancient Greek for hand.
In the two molecules on the right, the same four groups of atoms are joined to the central carbon atom, but they are arranged in different positions so that each stereoisomer is how the other would look reflected in a mirror.
These show the basic structure of amino acids, which are essential to life as they make proteins. In biochemistry, stereoisomers are called L when “left-handed” and D when “right-handed” (these labels come from “levorotatory” and “dextrorotatory”).
Unlike the C3H6O isomers above, these small stereoisomeric molecules have the same chemical properties. If we were to make them in the lab, we would expect to find an equal mixture of L- and D-type molecules. If we made proteins by joining these amino acids together, we would expect each chiral centre to randomly be L- or D-type, so the proteins would have many different stereoisomers.
In living creatures this is not the case. No one is certain why, but it turns out that almost all amino acids are L-type and sugars are D-type, so the chiral centres in the proteins and other big molecules made from these are all the same type – all the molecules have the same “handedness” or chirality, so they are called “homochiral.”
Researchers looking into the origin of life are keen to answer this question, as it would give us big clues as to how life started. If homochirality in simple molecules existed before life did, it would tell us a lot about the conditions on early Earth, but if it is a property of life itself, it could tell us how the first organisms formed.
There are many theories: some scientists think that in the early days of life, there was by random chance slightly more of one type, and chemical reactions which made molecules of the same chirality amplified this until one type dominated over the other.
Other scientists suggest that homochirality is an evolved trait: if the molecules of life were not homochiral, we would need two types of every enzyme to break down food – one for L type molecules and one for D type molecules, so a homochiral organism has an evolutionary advantage.
A further question is why the particular types we see today – L-amino acids and D-sugars – were favoured, rather than their stereoisomers. This may be due to purely-random chance, but one theory suggests that polarised light in space may have destroyed more D-amino acids in the icy dust and gas clouds in the solar system, leaving an excess of the L-type, which could have been delivered to Earth by meteorites or comets.
Scientists in labs around the world are trying to replicate the early Earth conditions and investigate the molecules that are found there, but some are looking even further afield: in 2014, the European Space Agency's Rosetta probe will deploy a lander down onto the surface of a comet, and look at the molecules within it2. If homochirality is found there, we will be a big step closer to understanding the origin of life on Earth.
This guest post, an explanation of homochirality and the search for the origin of life, is by Imperial College Department of Chemistry's sophomore student Keir Little.
References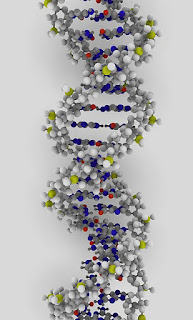 |
| Deoxyribonucleic acid (DNA) is a molecule which serves as the genetic blueprint for all known living organisms. Image credit: ynse (Creative Commons) |
To understand homochirality, we first need to look at the most basic building blocks that make up all lifeforms. The body of any living creature is made up of molecules – groups of atoms bonded together in a useful way. These range from the very small, such as water made from only three atoms, to the very large, such as the DNA strands that make up our genetic code.
Many of the big molecules in living creatures are made of smaller units joined together: starch, a carbohydrate found in food, is made from the sugar glucose, and proteins are made up of many different amino acids.
Molecules that have the same number and type of atoms but joined together in different ways are called “isomers” of each other. For example, depending on how the atoms are arranged, the C3H6O (that is, 3 carbon, 6 hydrogen and one oxygen atom) could be acetone, found in nail varnish remover, or methyl vinyl ether, used to make plastic, or also allyl alcohol used to make glycerol, which is found in many medicines and foodstuffs.
 |
| Isomers have the same chemical formula, but different layout of the constituent atoms. |
It's a special kind of isomerism, called stereoisomerism, that is important when discussing homochirality. Stereoisomerism can occur when a molecule has a carbon atom joined to four different groups. Remember that molecules aren't really flat like the drawings above, but have a three-dimensional structure. Stereoisomers have the same atoms bonded together in the same way, but are mirror images of each other. Think of your hands – the fingers, thumb and palm are joined together in the same way on each hand, but in opposite directions. In fact, molecules which can have stereoisomers are called “chiral,” from the ancient Greek for hand.
 |
| NASA scientists are examining meteorites for amino acids, to determine whether the L-type bias came from space. Image courtesy NASA |
These show the basic structure of amino acids, which are essential to life as they make proteins. In biochemistry, stereoisomers are called L when “left-handed” and D when “right-handed” (these labels come from “levorotatory” and “dextrorotatory”).
Unlike the C3H6O isomers above, these small stereoisomeric molecules have the same chemical properties. If we were to make them in the lab, we would expect to find an equal mixture of L- and D-type molecules. If we made proteins by joining these amino acids together, we would expect each chiral centre to randomly be L- or D-type, so the proteins would have many different stereoisomers.
In living creatures this is not the case. No one is certain why, but it turns out that almost all amino acids are L-type and sugars are D-type, so the chiral centres in the proteins and other big molecules made from these are all the same type – all the molecules have the same “handedness” or chirality, so they are called “homochiral.”
Researchers looking into the origin of life are keen to answer this question, as it would give us big clues as to how life started. If homochirality in simple molecules existed before life did, it would tell us a lot about the conditions on early Earth, but if it is a property of life itself, it could tell us how the first organisms formed.
There are many theories: some scientists think that in the early days of life, there was by random chance slightly more of one type, and chemical reactions which made molecules of the same chirality amplified this until one type dominated over the other.
Other scientists suggest that homochirality is an evolved trait: if the molecules of life were not homochiral, we would need two types of every enzyme to break down food – one for L type molecules and one for D type molecules, so a homochiral organism has an evolutionary advantage.
A further question is why the particular types we see today – L-amino acids and D-sugars – were favoured, rather than their stereoisomers. This may be due to purely-random chance, but one theory suggests that polarised light in space may have destroyed more D-amino acids in the icy dust and gas clouds in the solar system, leaving an excess of the L-type, which could have been delivered to Earth by meteorites or comets.
 |
| The COSAC experiment, aboard the Rosetta mission's Philae lander, is designed to identify organic molecules in cometary material.1 Image credit: ESA |
This guest post, an explanation of homochirality and the search for the origin of life, is by Imperial College Department of Chemistry's sophomore student Keir Little.
why don't all references have links?
1 Goesmann, Fred. et al, COSAC, THE COMETARY SAMPLING AND COMPOSITION EXPERIMENT ON PHILAE.Space Science Reviews (2007) 128: 257–280 DOI: 10.1007/s11214-006-9000-6
2 David, Cline. On the physical origin of the homochirality of life. European Review Vol 13, Supp No. 2 P49-59. Academica Europaea, 2005.

No comments:
Post a Comment