There is a disease that you probably haven’t heard of, which infects 35-50 million people every year - mainly in areas of developing countries where wastewater isn’t kept separate from drinking water. It causes symptoms ranging from stomach cramps to life-threatening dysentery (bloody diarrhoea) and, in extreme cases, fatal liver abscesses. This disease, called Amoebiasis, kills up to 100,000 people annually, and is caused by the parasitic microbe Entamoeba histolytica.
When accidentally ingested by a human, the parasites stay as ‘armoured’ dormant cells called cysts, until they pass through the highly acidic stomach. Upon entering the large intestine, the environment becomes more hospitable for them and allows them to transform into their active form.
Many of them will stay in the intestines but some pass out of the host in faeces, and transform into cysts again in order to survive in the more varied temperature and acidity levels they must endure before finding another host. But it is those that remain which (might) cause a problem. This is the main mystery of E. histolytica - not everybody infected will experience the same symptoms, if any. So what triggers the different levels of infection?
The parasites can survive in a human intestine for a long time without causing any symptoms at all, as they ‘swim’ around eating passing bacteria and food. However, not all E. histolytica are the same - some strains (the microbial equivalent of dog breeds) are termed ‘pathogenic’, which means they cause disease. But this classification is far from straight forward.
The most common symptoms of Amoebiasis are diarrhoea and dysentery, caused by the parasites attacking and destroying the lining of the intestines. The majority of cases don’t progress any further than this, and can be treated with antibiotics, but on rare occasions the parasites burrow into the bloodstream. When this happens, they’re carried to another bodily organ - usually the liver, as it’s the first organ the bloodstream reaches. Once the liver is infected abscesses form, which destroys the vital organ and kills the victim. Infections have also been known to take hold in the lungs, and the brain.
Confusingly, just because a strain causes Amoebiasis in one person doesn’t mean that it will definitely cause the disease in another. So when we say that a strain of E. histolytica is pathogenic, we mean that it is known to have the potential to cause disease, not that it always will.
So how do they cause disease? On their surfaces, the parasites have ‘lectins’; proteins that bind to sugars on the surfaces of the host cells, allowing them to stick to the lining of the intestine, where they begin causing damage. But other harmless species related to E. histolytica also have this lectin, so it’s unlikely to be the sole trigger for the entire disease. Two other proteins are thought to play a role here too, but we don’t know much about them. It’s possible that sticking to host cells is part of the disease trigger, and given that the disease doesn’t always take hold straight away, even when the parasites are capable, it’s likely that this stage is kick-started by something else we’re yet to discover.
Once stuck to the intestine wall, the parasites spit out enzymes called ‘cysteine proteases’, which cut in-between the cells of the host's intestine like scissors, to break up the lining. The intestine wall has millions of tiny folds in it to increase the surface area across which water can be absorbed. The cysteine proteases destroy these folds, resulting in more water passing out in the faeces, causing diarrhoea.
Some reports suggest that pathogenic strains of the parasite possess genes that harmless strains don’t and that these could encode different versions these cysteine proteases. Others, meanwhile, have found that the various strains make these potentially harmful proteins at different times and in different amounts, which could explain the disparity.
Some disease-causing infections don’t get any worse than this, but others can progress to cause dysentery. In these cases, the parasites unleash a veritable arsenal of weapons and defences to both destroy, and defend against, host cells. It may be that only strains that possess such armaments are capable of causing dysentery, but as with many stages of Amoebiasis this is a grey area.
As E. histolytica destroys cells in the wall of the intestine, it releases chemical signals that attract cells of the host’s immune system, calling them to the site of infection. When they arrive, the white blood cells assault the parasites and the surrounding host cells in an effort to prevent the infection spreading.
Unfortunately for us, the invaders have a range of counter-measures for the immune system’s weapons, meaning that the intestine walls suffer more damage than they do. This clears the way for the parasites to move deeper into the wall and increases the amount of blood lost into the faeces.
It is difficult to see how the ability to infect organs other than the intestine could have evolved in E. histolytica, as these infections do not seem to be beneficial for them. Diarrhoea and dysentery improve the chances of the parasite being passed on to a new host. However, if a liver infection kills the host then it stops the parasite being passed on. This course of action leads to a literal dead end for E. histolytica unless it helps it in some way that we don’t yet understand. As you might imagine, scientists are still trying to work out why this happens.
One possibility is that breaking into the bloodstream is entirely accidental. By doing so, the parasites are entering an environment that really doesn’t suit them at all. E. histolytica is an anaerobe, meaning that it is best suited to environments containing no oxygen, but the blood is filled with it. This can destroy the parasites, so they must use some of the same sets of proteins that they use to defend against the immune system’s cells if they’re to have any hope of surviving.
It’s likely that only the best-defended strains can survive in the bloodstream. Any that aren’t well protected will be destroyed, theoretically leaving only the most resilient parasites to invade other bodily organs.
Different people infected with the same strain of E. histolytica can suffer from different symptoms, if they suffer at all, and some hosts can be infected for months without showing signs of the disease before suddenly developing symptoms. This means it’s entirely possible that possession of genes which code for certain proteins is not enough. Instead, certain interactions between host and parasite may trigger the various disease states. Unfortunately, this is a relatively new school of thought so not much work has been carried out so far. Certainly though, it is an exciting avenue of research.
Whilst we don’t know what triggers the different levels of infection exactly, a lot of work has been carried out in recent years to identify potential triggers. As we learn more about this unpredictable and versatile parasite, we’ll come closer to discovering what turns it into a killer. Certainly, we know of many of the genes and proteins E. histolytica uses to attack us; we just don’t know what starts it off down that path.
We’ve seen that it uses the host’s immune system to its advantage to move deeper into the intestine wall. We’ve also seen that, whilst we attempt to label strains as ‘pathogenic’ and ‘non-pathogenic’, the parasites don’t always do what we expect in every case! Could it be interactions between host and parasite that trigger the different disease stages? Unfortunately, without taking DNA samples from every patient tested for the disease (an ethical, as well as practical, challenge) it could take time to identify any responsible human proteins and host-parasite interactions. Whilst several research groups are tackling this monumental task, many others are studying groups of E. histolytica genes and proteins. Some are comparing the amounts of certain proteins that supposedly ‘pathogenic’ strains produce, compared with ‘harmless’ strains. Others aim to understand how the genes and their proteins work, in order to discover the mechanisms behind some of the key stages in the disease. If we know exactly how symptoms are caused, and which proteins are key players in disease, we may be able to improve treatments.
Of course, the ultimate dream is to develop a preventative treatment for Amoebiasis, rather than a cure. This is the Holy Grail of any disease research. However, given the complexities of this particular disease, this may be a long time coming. Until then, the best we can hope to do is improve our ability to diagnose and treat patients and to prevent the spread of this prolific killer.
Ian Wilson is in the fourth and final year of his PhD studying the genetics of Entamoeba histolytica. He is based at the University of Liverpool, which is also where he completed his undergraduate degree in Microbiology. When he’s not working on his project, Ian writes for various science communication websites, including his own blog, The Science Gremlin, with the intention of becoming a full-time science communicator when he graduates. You can get in touch with Ian and keep up to date with his writing by following him on Twitter at @Science_Gremlin.
When accidentally ingested by a human, the parasites stay as ‘armoured’ dormant cells called cysts, until they pass through the highly acidic stomach. Upon entering the large intestine, the environment becomes more hospitable for them and allows them to transform into their active form.
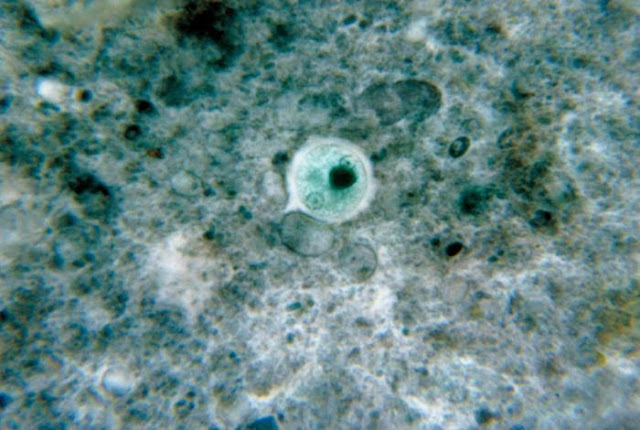 |
| It was once thought that E. Histolytica infected 10% of the world's population - but although that figure has been reduced to just 1%, that's still a lot of people. Image credit: CDC / Dr. George Healy (1964) |
Many of them will stay in the intestines but some pass out of the host in faeces, and transform into cysts again in order to survive in the more varied temperature and acidity levels they must endure before finding another host. But it is those that remain which (might) cause a problem. This is the main mystery of E. histolytica - not everybody infected will experience the same symptoms, if any. So what triggers the different levels of infection?
The parasites can survive in a human intestine for a long time without causing any symptoms at all, as they ‘swim’ around eating passing bacteria and food. However, not all E. histolytica are the same - some strains (the microbial equivalent of dog breeds) are termed ‘pathogenic’, which means they cause disease. But this classification is far from straight forward.
The most common symptoms of Amoebiasis are diarrhoea and dysentery, caused by the parasites attacking and destroying the lining of the intestines. The majority of cases don’t progress any further than this, and can be treated with antibiotics, but on rare occasions the parasites burrow into the bloodstream. When this happens, they’re carried to another bodily organ - usually the liver, as it’s the first organ the bloodstream reaches. Once the liver is infected abscesses form, which destroys the vital organ and kills the victim. Infections have also been known to take hold in the lungs, and the brain.
 |
| Image copyright Rachael Tremlett / Things We Don't Know |
Confusingly, just because a strain causes Amoebiasis in one person doesn’t mean that it will definitely cause the disease in another. So when we say that a strain of E. histolytica is pathogenic, we mean that it is known to have the potential to cause disease, not that it always will.
Why don't pathogenic strains always cause disease?
So how do they cause disease? On their surfaces, the parasites have ‘lectins’; proteins that bind to sugars on the surfaces of the host cells, allowing them to stick to the lining of the intestine, where they begin causing damage. But other harmless species related to E. histolytica also have this lectin, so it’s unlikely to be the sole trigger for the entire disease. Two other proteins are thought to play a role here too, but we don’t know much about them. It’s possible that sticking to host cells is part of the disease trigger, and given that the disease doesn’t always take hold straight away, even when the parasites are capable, it’s likely that this stage is kick-started by something else we’re yet to discover.
 |
| No intestinal parasites please. |
Some reports suggest that pathogenic strains of the parasite possess genes that harmless strains don’t and that these could encode different versions these cysteine proteases. Others, meanwhile, have found that the various strains make these potentially harmful proteins at different times and in different amounts, which could explain the disparity.
Some disease-causing infections don’t get any worse than this, but others can progress to cause dysentery. In these cases, the parasites unleash a veritable arsenal of weapons and defences to both destroy, and defend against, host cells. It may be that only strains that possess such armaments are capable of causing dysentery, but as with many stages of Amoebiasis this is a grey area.
As E. histolytica destroys cells in the wall of the intestine, it releases chemical signals that attract cells of the host’s immune system, calling them to the site of infection. When they arrive, the white blood cells assault the parasites and the surrounding host cells in an effort to prevent the infection spreading.
Unfortunately for us, the invaders have a range of counter-measures for the immune system’s weapons, meaning that the intestine walls suffer more damage than they do. This clears the way for the parasites to move deeper into the wall and increases the amount of blood lost into the faeces.
Is sticking to host cells part of the disease trigger?
 |
| A researcher in the bio lab at Walter Reed Army Institute of Research in Silver Spring, Md. Image credit: US Army Africa |
One possibility is that breaking into the bloodstream is entirely accidental. By doing so, the parasites are entering an environment that really doesn’t suit them at all. E. histolytica is an anaerobe, meaning that it is best suited to environments containing no oxygen, but the blood is filled with it. This can destroy the parasites, so they must use some of the same sets of proteins that they use to defend against the immune system’s cells if they’re to have any hope of surviving.
It’s likely that only the best-defended strains can survive in the bloodstream. Any that aren’t well protected will be destroyed, theoretically leaving only the most resilient parasites to invade other bodily organs.
What triggers the different levels of infection?
Different people infected with the same strain of E. histolytica can suffer from different symptoms, if they suffer at all, and some hosts can be infected for months without showing signs of the disease before suddenly developing symptoms. This means it’s entirely possible that possession of genes which code for certain proteins is not enough. Instead, certain interactions between host and parasite may trigger the various disease states. Unfortunately, this is a relatively new school of thought so not much work has been carried out so far. Certainly though, it is an exciting avenue of research.
Whilst we don’t know what triggers the different levels of infection exactly, a lot of work has been carried out in recent years to identify potential triggers. As we learn more about this unpredictable and versatile parasite, we’ll come closer to discovering what turns it into a killer. Certainly, we know of many of the genes and proteins E. histolytica uses to attack us; we just don’t know what starts it off down that path.
We’ve seen that it uses the host’s immune system to its advantage to move deeper into the intestine wall. We’ve also seen that, whilst we attempt to label strains as ‘pathogenic’ and ‘non-pathogenic’, the parasites don’t always do what we expect in every case! Could it be interactions between host and parasite that trigger the different disease stages? Unfortunately, without taking DNA samples from every patient tested for the disease (an ethical, as well as practical, challenge) it could take time to identify any responsible human proteins and host-parasite interactions. Whilst several research groups are tackling this monumental task, many others are studying groups of E. histolytica genes and proteins. Some are comparing the amounts of certain proteins that supposedly ‘pathogenic’ strains produce, compared with ‘harmless’ strains. Others aim to understand how the genes and their proteins work, in order to discover the mechanisms behind some of the key stages in the disease. If we know exactly how symptoms are caused, and which proteins are key players in disease, we may be able to improve treatments.
Of course, the ultimate dream is to develop a preventative treatment for Amoebiasis, rather than a cure. This is the Holy Grail of any disease research. However, given the complexities of this particular disease, this may be a long time coming. Until then, the best we can hope to do is improve our ability to diagnose and treat patients and to prevent the spread of this prolific killer.
Ian Wilson is in the fourth and final year of his PhD studying the genetics of Entamoeba histolytica. He is based at the University of Liverpool, which is also where he completed his undergraduate degree in Microbiology. When he’s not working on his project, Ian writes for various science communication websites, including his own blog, The Science Gremlin, with the intention of becoming a full-time science communicator when he graduates. You can get in touch with Ian and keep up to date with his writing by following him on Twitter at @Science_Gremlin.
References
why don't all references have links?
[1] Stanley SJ. Amoebiasis. The Lancet 2003; 361: 1025-1034.
[2] Loftus B, Anderson I, Davies R et al. The genome of the protist parasite Entamoeba histolytica. Nature 2005; 433: 865-868. DOI: 10.1038/nature03291
[3] Clark CG, Alsmark, UCM, Tazreiter M et al. Structure and content of the Entamoeba histolytica genome. Advances in Parasitology 2007: 65: 51–190.
[4] Lorenzi HA, Puiu D, Miller JR et al. New assembly, reannotation and analysis of the Entamoeba histolytica genome reveal new genomic features and protein content information. PLoS Neglected Tropical Diseases 2010; 4: e716. DOI: 10.1371/journal.pntd.0000716
[5] Blessmann J, Van LP, Nu PAT et al. Epidemiology of amebiasis in a region of high incidence of amebic liver abscess in central Vietnam. The American Journal of Tropical Medicine and Hygiene 2002; 66: 578-583. PMID: 12201594
[6] Petri WA, Haque R & Mann BJ. The bittersweet interface of parasite and host: lectin-carbohydrate interactions during human invasion by the parasite Entamoeba histolytica. Annual Review of Microbiology 2002; 56: 39-64. PMID: 12142490
[7] Lidell ME, Moncada DM, Chadee K & Hansson GC. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proceedings of the National Academy of Sciences of the United States of America 2006; 103: 9298-9303. DOI: 10.1073/pnas.0600623103
[8] Lejeune M, Rybicka JM & Chadee K. Recent discoveries in the pathogenesis and immune response toward Entamoeba histolytica. Future Microbiology 2009; 4: 105-118. PMID: 19207103
[9] Guo X, Houpt E & Petri WA. Crosstalk at the initial encounter: interplay between host defense and ameba survival strategies. Current Opinion in Immunology 2007; 19: 376-384. DOI: 10.1016/j.coi.2007.07.005
[10] Santi-Rocca J, Rigothier M-C & Guillén N. Host-microbe interactions and defense mechanisms in the development of amoebic liver abscesses. Clinical Microbiology Reviews 2009; 22: 65-75. DOI: 10.1128/CMR.00029-08
[11] Bruchhaus I, Roeder T, Lotter H, Schwerdtfeger M & Tannich E. Differential gene expression in Entamoeba histolytica isolated from amoebic liver abscess. Molecular Microbiology 2002; 44: 1063-1072. DOI: 10.1046/j.1365-2958.2002.02941.x PMID: 12010498
[12] Gilchrist CA & Petri WA. Using differential gene expression to study Entamoeba histolytica pathogenesis. Trends in Parasitology 2009; 25: 124-131. DOI: 10.1016/j.pt.2008.12.007
[13] Leitsch D, Wilson IB, Paschinger K & Duchêne M. Comparison of the proteome profiles of Entamoeba histolytica and its close but non-pathogenic relative Entamoeba dispar. The Middle European Journal of Medicine 2006; 118 (Suppl 3): 37-41. PMID: 17131239
[14] Biller L, Davis PH, Tillack M et al. Differences in the transcriptome signatures of two genetically related Entamoeba histolytica cell lines derived from the same isolate with different pathogenic properties. BMC Genomics 2010; 11. DOI: 10.1186/1471-2164-11-63 PMID: 20102605
[15] Davis PH, Schulze J & Stanley SL. Transcriptomic comparison of two Entamoeba histolytica strains with defined virulence phenotypes identifies new virulence factor candidates and key differences in the expression patterns of cysteine proteases, lectin light chains, and calmodulin. Molecular and Biochemical Parasitology 2007; 151: 118-128. DOI:10.1016/j.molbiopara.2006.10.014
[16] MacFarlane RC & Singh U. Identification of differentially expressed genes in virulent and nonvirulent Entamoeba Species: Potential implications for amebic pathogenesis. Infection and Immunity 2006; 74: 340-351. DOI: 10.1128/IAI.74.1.340-351.2006
[17] Biller L, Schmidt H, Krause E et al. Comparison of two genetically related Entamoeba histolytica cell lines derived from the same isolate with different pathogenic properties. Proteomics 2009; 9: 4107-4120. PMID: 19688750

No comments:
Post a Comment